Anti-oxidative Potential of Essential Oil of Rosmarinus officinalis in Cadmium-induced Neurotoxicity on the Cerebellum of Adult Wistar Rats
Main Article Content
Abstract
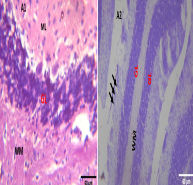
The cerebellum connects the brain’s sensory areas to its motor areas and is required to coordinate fine movements. However, it is vulnerable to neurotoxic agents and poisoning. This study aimed to determine the role of rosemary essential oil in cadmium-induced toxicity in the cerebellum of adult Wistar rats. Twenty-five (25) male Wistar rats weighing between 120 and 160 g were separated into five (5) groups, consisting of five (5) rats in each group. Group A (Control), Group B (6 mg/kg of Cadmium chloride), Group C (6 mg/kg of CdCl2 + 200 mg/kg of Essential oil of Rosmarinus officinalis (EORO), Group D (6 mg/kg of CdCl2 + 300 mg/kg of EORO), and Group E (6 mg/kg of CdCl2 + 400 mg/kg of EORO). Neuro-behavioural assessment studies (open field test), brain’s histological studies, and biochemical assessment of total cholesterol (TC), triacylglycerol (TG), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) were carried out. Using one-way ANOVA and paired student’s t-test, both the statistical differences in the group’s mean and its comparison were analysed with the generated result reported as mean ± standard error of mean (SEM). Cadmium chloride caused locomotive impairment. These were demonstrated by distorted pia matter, molecular layer, and granular layer and haemorrhage around the neuronal cells. Neuro-behavioural analysis showed line crossing (LC) and freezing duration (FD) as (46.50±1.50, 17.50±2.50), (24.00±12.42, 181.00±56.07), (43.00±3.00, 72.50±2.50), (34.67±6.36, 66.67±8.82), and (34.67±8.82, 79.00±14.64) for groups A, B, C, D and E respectively. However, concurrent administration of rosemary essential oil ameliorates the neurotoxic effects induced by cadmium.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Fine EJ, Ionita CC, Lohr L. The history of the development of the cerebellar examination. Semin Neurol. 2002; 22(4):375-384
Manto M. Toxic agents causing cerebellar ataxias. Handb Clin Neurol. 2012; 103:201-213.
Manto, M. Cerebellotoxic agents. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi, N. (eds) HC and CD. 2013; 2079-2117.
Zheng W, Perry DF, Nelson DL, Aposhian HV. Choroid plexus protects cerebrospinal fluid against toxic metals. The FASEB Journal. 1991; 5(8):2188-2930.
Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008; 108(2):177-184.
Karoui-Kharrat D, Kaddour H, Hamdi Y, Mokni M, Amri M, Mezghani S. Response of antioxidant enzymes to cadmium-induced cytotoxicity in rat cerebellar granule neurons. Open Life Sci. 2017; 12(1): 113-119. https://doi.org/10.1515/biol-2017-0013
Wahdan MH, Ismail AK, Saad MF, Meguid EMA, Rashidy AH, Rana MF. Effect of cadmium exposure on the structure of the cerebellar vermis of growing male albino rat. Intl Res J Appl and Basic Sci. 2014; 8(2):163–176.
Jacopo J, Valerio B, Gabriele M, Alessandra P. Cadmium-induced neurotoxicity: still much ado. Neural Regen Res. 2018; 13(11): 1879–1882.
Hcini K, Sotomayor JA, Jordan MJ, Bouzid S. Chemical Composition of the Essential Oil of Rosemary (Rosmarinus officinalis L.) of Tunisian Origin. Asian J. Chem. 2012; 25(5):2601–2603.
Chalchat JC, Garry RP, Michet A, Benjilali B, Chabart JL. Essential oils of rosemary (Rosmarinus officinalis L). The chemical composition of oils of various origins (Morocco, Spain, France). J. Essent. Oil Res.. 1993; 5(6):613-618
Domokos J, Héthelyi É, Pálinkás J, Szirmai S, Tulok HM. Essential Oil of Rosemary (Rosmarinus officinalis L.) of Hungarian Origin. J. Essent. Oil Res...1997; 9(1):41-45
Raphaelle SB, Brenda LSO, Arlindo CMP, Hady K, José CTC. Rosmarinus officinalis essential oil: A review of its phytochemistry, antiinflammatory activity, and mechanisms of action involved. J. Ethnopharm. 2019; 229: 29-45
Wada M. Evaluation of quenching effects of non-water-soluble and water-soluble rosemary extracts against active oxygen species by chemiluminescent assay. Food Chem. 2004; 87(2): 261–267.
Pintore, G., M. Usai, P. Bradesi, C. Juliano, G. Batto, F. Tomi, M. Chessa, R. Cerri and J. Casanova. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from sardinia and corsica. Flavor Fragr, J. 2002; 17(1): 15-19
Slamenova D, Kuboskova K, Horvathova E, Robichova S. Rosemary-stimulated reduction of DNA strand breaks and FPG-sensitive sites in mammalian cells treated with H2O2 or visible light-excited Methylene Blue. Ca Letters. 2002;177(2):145–153.
Socaci, S. A., T. Maria, S. Carmen a HFS992520nd D.S. Varban. Preliminary study on rosemary essential oil. J. Agric. Plant Technol. 2008; 14(1): 128-132
Jamshidi R, Afzalim Z, Afzali D. Chemical Composition of Hydrodistillation Essential Oil of Rosemary in Different Origins in Iran and Comparison with Other Countries. Am-Euras. J. Agric. & Environ. Sci. 2009; 5(1): 78-81
Rakha EA, El-Sayed ME, Menon S, Green AR, Lee AH, Ellis IO. Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2008; 111(1):121-127.
Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutri Res. 2002; 22(9):1041–1047.
Gheldof N, Engeseth NJ. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J Agric Food Chem. 2002; 50(10):3050-3055.
Brown TB, Lovato LM, Parker D. Procedural sedation in the acute care setting. Am Fam Physician. 2005; 71(1): 85-90.
Crusio WE. Genetic dissection of mouse exploratory behaviour. Behav Brain Res. 2001; 125(1-2): 127-132. doi: 10.1016/s0166-4328(01)00280-7. PMID: 11682103.
Sturman O, Germain PL, Bohacek J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress; 2018; 21(5): 443-452.
Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983; 29(6):1075-1080.
Negele JC, Dotson DG, Liu W, Sweeney HL, Putkey JA. Mutation of the high affinity calcium binding sites in cardiac troponin C. J Biol Chem. 1992; 267(2):825-831.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502.
Sheehan DC and Hrapchak BB. Theory and Practice of Histotechnology. 2nd ed; Battelle Pr, ISBN 10: 1987; 1574770675 ISBN 13: 9781574770674.
Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess. 2019; 191(7):419.
Brzóska MM, Kamiński M, Supernak-Bobko D, Zwierz K, Moniuszko-Jakoniuk J. Changes in the structure and function of the kidney of rats chronically exposed to cadmium. I. Biochemical and histopathological studies. Arc of Toxic. 2003; 77(6): 344–352.
Poli V, Madduru R, Aparna Y, Kandukuri V, Motireddy SR. Amelioration of Cadmium-Induced Oxidative Damage in Wistar Rats by Vitamin C, Zinc and N-Acetylcysteine. Med Sci (Basel). 2022; 10(1):7.
Renuka M, Aparna Y, Venkataramanaiah P, Reddy MS. Vitamin C, E and Zinc ameliorates cadmium-toxicity induced biochemical changes in male Albino rats. Toxicol Forensic Med Open J. 2021; 6(1): 13-19. doi: 10.17140/TFMOJ-6-137
Renugadevi J, Prabu SM. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol. 2010; 62(5):471-481.
Young JL, Yan X, Xu J, Yin X, Zhang X, Arteel GE, Barnes GN, States JC, Watson WH, Kong M, Cai L, Freedman JH. Cadmium and High-Fat Diet Disrupt Renal, Cardiac and Hepatic Essential Metals. Sci Rep. 2019; 9(1):14675.
Seyidoglu N, Koseli E, Gurbanli R, Aydin C. Role of essential oils in antioxidant capacity and immunity in a rat model of mixed stress. S. Afr. j. anim. sci. 2021; 51(4): 426-436.
Basheer AJ, Al-Hayyali FQM, Al-Kassim NAH. Effect of different doses of volatile oil extract of rosemary in suckling rats on some physiological parameters and pups. Al-Qadisiyah J. Dairy Food Sci. Res.2023; 42(4): 522-528.
Abou-Hashem AAM. Evaluation of the rodenticidal effects of some plant extracts under laboratory and field conditions. J of Basic & App Zoo. 2012; 65(5):282–288.
Samarghandian S, Azimi-Nezhad M, Shabestari MM, Azad FJ, Farkhondeh T, Bafandeh F. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscip Toxicol. 2015; 8(3):151-154.
Chen L, Liu L, Luo Y, Huang S. MAPK and mTOR pathways are involved in cadmium‐induced neuronal apoptosis. J of Neurochem. 2007; 105(1): 251–261.
Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L. Rosemary (Rosmarinus officinalis) essential oil components exhibit anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects in experimental diabetes. Pathophysiology. 2017; 24(4):297-303.
Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015; (96):e52434.
Abdulmajeed WI, Sulieman HB, Zubayr MO, Imam A, Amin A, Biliaminu SA, Owoyele BV. Honey prevents neurobehavioural deficit and oxidative stress induced by lead acetate exposure in male wistar rats- a preliminary study. Metab Brain Dis. 2016; 31(1):37–44.
Adaeze BE, Churchill AI. Cadmium and mercury exposure: oxidative, neurobehavioural and histological alterations to the cerebellum of wistar rats. Int. Med. J. 2022; 15(2): 141–147.
Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001; 23(5):489-495.
Montgomery KS, Mackey J, Thuett K, Ginestra S, Bizon JL, Abbott LC. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behav Brain Res. 2008; 191(1):55-61.
Abubakar K, Mailafiya MM, Danmaigoro A, Chiroma SM, Rahim EBA, Zakaria MZ. Curcumin attenuates Lead-Induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules. 2019; 9(9):453.
Dogra S, Khanna AK, Kaw JL. Antibody forming cell response to nickel and nickel-coated fly ash in rats. Hum Exp Toxicol. 1999; 18(5):333-337. doi: 10.1191/096032799678840183. PMID: 10372756.
Kouadria M, Djemli S, Tahraoui A. The protective effect of Zinc and Magnesium against subchronic Cadmium toxicity in Wistar rats (Biochemical and neurobehavioral effects). Asian J Pharm Clin Res [Internet]. 2019 May 7; 12(5):217-225.
Lamtai M, Chaibat J, Ouakki S, Zghari O, Mesfioui A, El Hessni A, Rifi EH, Marmouzi I, Essamri A, Ouichou A. Effect of Chronic Administration of Nickel on Affective and Cognitive Behavior in Male and Female Rats: Possible Implication of Oxidative Stress Pathway. Brain Sci. 2018; 8(8):141.
Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008; 104(4):1116-1131.
Ibáñez C, Valdés A, García-Cañas V, Simó C, Celebier M, Rocamora-Reverte L, Gómez-Martínez A, Herrero M, Castro-Puyana M, Segura-Carretero A, Ibáñez E, Ferragut JA, Cifuentes A. Global Foodomics strategy to investigate the health benefits of dietary constituents. J Chromatogr A. 2012; 1248:139-153.
Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: A review. Eur J Med Chem. 2019; 178:687-704.
Eddin LB, Jha NK, Meeran MFN, Kesari KK, Beiram R, Ojha S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules. 2021; 26(15):4535.
Mohamed HW, Ayman KI, Mohamed FS, Eiman MM, Ahmed HR, Mujtaba FR. Effect of cadmium Exposure on structure of cerebellar vermis of growing male Albino rat. Int. Res. J. Appl. Basic Sci2014;8 (2):163-176