Insulin Secretion and Glucose Uptake Enhancement by Mimosa pudica and Abutilon indicum: Potential Antidiabetic Therapy
Main Article Content
Abstract
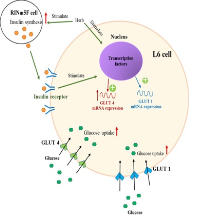
Mimosa pudica (M) and Abutilon indicum (A) are used as an antidiabetic mixture at a ratio of 1:1 (MA11)in traditional Thai medicine. However, the mechanisms by which these plants exhibit antidiabetic effects have not been clearly elucidated. In this study, RINm5F insulinoma cells were treated with 6 mM streptozotocin (STZ) for 1 h to induce cellular damage and generate an in vitro model of diabetes. Extracts from M and A and mixtures of M and A extracts contain several phytochemicals, including flavonoids, tannins and saponins. All the extract samples at a concentration of 1,000 µg/mL exhibited antioxidant activity without cytotoxicity to RINm5F and L6 myotube cells. Pretreatment with M or A or with a mixture of the two prevented STZ-induced RINm5F cell death in a concentration-dependent manner. The greatest recovery effect was found in MA12, followed by MA11 and MA21. In addition, the M and A extracts and the mixtures of M and A extracts (100,000 mg/mL) exhibited insulin secretory activity in RINm5F cells that was similar to that of glibenclamide. Furthermore, all the plant extracts induced glucose uptake in L6 myotube cells. The induction of glucose uptake by M, A, and mixtures of M and A was derived from the upregulation of GLUT1 and GLUT4 synthesis in L6 myotube cells. These results indicate that M. pudica and A. indicum are potential sources of antidiabetic agents. In conclusion, the antidiabetic formula of M and A at a ratio of 2:1 (MA21) has the greatest potential for therapeutic use as an antidiabetic herbal mixture.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
1. American Diabetes Association, Standards of medical care in diabetes-2009. Diabetes Care. 2009; 32(Suppl 1): S13.
2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87:4-14.
3. Georgia S, Bhushan A. β cell replication is the primary mechanism for maintaining postnatal β cell mass. J Clin Invest. 2011; 14:963-968.
4. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008; 57:1584-1594.
5. Wang J, Wang H. Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev. 2017; 1(2017):1930261.
6. Hosseini A, Shafiee-Nick R, Ghorbani A. Pancreatic beta cell protection/regeneration with phytotherapy. Braz J Pharm Sci. 2015; 51:1-16.
7. Li N, Frigerio F, Maechler P. The sensitivity of pancreatic β-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans. 2008; 36:930-934.
8. Sharma RB, O’Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Alonso LC. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest. 2015; 125:3831-3846.
9. Aboonabi A, Rahmat A, Othman F. Antioxidant effect of pomegranate against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Toxicol Rep. 2014; 1:915-922.
10. West I. Radicals and oxidative stress in diabetes. Diabetic Med. 2000; 17:171-180.
11. Nasri H, Shirzad H, Baradaran A, Rafieian-Kopaei M. Antioxidant plants and diabetes mellitus. J Res Med Sci. 2015; 20:491.
12. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996; 20: 933-956.
13. Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents, Phytomedicine. 1995; 2:137-189.
14. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107: 1058-1070.
15. Parekh J, Chanda S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr J Biomed Res. 2007; 10: 175-181.
16. Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K. Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res. 2008; 102:981-988.
17. Krisanapun C, Lee SH, Peungvicha P, Temsiririrkkul R, Baek SJ. Antidiabetic activities of Abutilon indicum (L.) sweet are mediated by enhancement of adipocyte differentiation and activation of the GLUT1 promoter. Evid Based Complement Alternat Med. 2011; 1(2011): 167684.
18. Tunna TS, Ahmed QU, Uddin A, Sarker M, Islam Z. Weeds as alternative useful medicinal source: Mimosa pudica Linn. on diabetes mellitus and its complications. Adv Mat Res. 2014; 995:49-59.
19. Tunna T, Zaidul I, Ahmed Q, Ghafoor K, Al-Juhaimi F, Uddin M, Ferdous S. Analyses and profiling of extract and fractions of neglected weed Mimosa pudica Linn. traditionally used in Southeast Asia to treat diabetes. S Afr J Bot. 2015; 99:144-152.
20. Muhammad G, Hussain MA, Jantan I, Bukhari SNA. Mimosa pudica L. A high‐value medicinal plant as a source of bioactives for pharmaceuticals. Compr Rev Food Sci Food Saf. 2016; 15: 303-315.
21. Parasuraman S, Ching TH, Leong CH, Banik U. Antidiabetic and antihyperlipidemic effects of a methanolic extract of Mimosa pudica (Fabaceae) in diabetic rats. Egypt J Basic Appl Sci. 2019; 6:137-148.
22. Konsue A, Talubmook C. Effect of Thai folklore recipe from Abutilon indicum and Mimosa pudica in streptozotocin-induced diabetic rats. Pharmacogn J. 2018; 10:480-485.
23. Rajendran R, Hemachander R, Ezhilarasan T, Keerthana C, Saroja D, Saichand K, Abdullah MG. Phytochemical analysis and in-vitro antioxidant activity of Mimosa pudica Lin., leaves. Res J Pharm Technol. 2012; 3:551-555.
24. Wang J, Yue YD, Tang F, Sun J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa textilis McClure. Molecules. 2012; 17:12297-12311.
25. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987; 47:936-942.
26. Wang N, Zhang J, Qin M, Yi W, Yu S, Chen Y, Zhang R. Amelioration of streptozotocin‑induced pancreatic β cell damage by morin: Involvement of the AMPK‑FOXO3‑catalase signaling pathway. Int J Mol Med. 2018; 41: 1409-1418.
27. Yusoff NA, Lim V, Al-Hindi B, Abdul Razak KN, Widyawati T, Anggraini DR, Ahmad M, Asmawi MZ. Nypa fruticans Wurmb vinegar's aqueous extract stimulates insulin secretion and exerts hepatoprotective effect on STZ-induced diabetic rats. Nutrients. 2017; 9:925.
28. Inthongkaew P, Chatsumpun N, Supasuteekul C, Kitisripanya T, Putalun W, Likhitwitayawuid K, Sritularak B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev Bras Farmacogn. 2017; 27:480-487.
29. Li B, Kim DS, Yadav RK, Kim HR, Chae HJ. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int J Mol Med. 2015; 36: 53-64.
30. Mangmool S, Kunpukpong I, Kitphati W, Anantachoke N. Antioxidant and anticholinesterase activities of extracts and phytochemicals of Syzygium antisepticum leaves. Molecules. 2021; 26: 3295.
31. Mangmool S, Denkaew T, Parichatikanond W, Kurose H. b-Adrenergic receptor and insulin resistance in the heart. Biomol Ther. 2017; 25:44-56.
32. Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. 2000; 21:585-618.
33. Harrigan RA, Nathan MS, Beattie P. Oral agents for the treatment of type 2 diabetes mellitus: pharmacology, toxicity, and treatment. Ann Emerg Med. 2001; 38: 68-78.
34. Wang J, Wang H. Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev. 2017; 1(2017):1930261.
35. Prasad CN, Anjana T, Banerji A, Gopalakrishnapillai A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010; 5:531-536.
36. Gandhi GR, Jothi G, Antony PJ, Balakrishna K, Paulraj MG, Ignacimuthu S, Stalin A, Al-Dhabi NA. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. 2014; 15:201-216.
37. Wong TS, Mohamed TapF, Hashim Z, Abdul Majid FA, Zakaria NH, Siahaan P, Mogadem A. Dual actions of gallic acid and andrographolide trigger AdipoR1 to stimulate insulin secretion in a streptozotocin-induced diabetes rat model. J Tradit Complement Med. 2022; 13: 11-19.
38. Liu ZH, Li B. Procyanidin B1 and p-coumaric acid from highland barley grain showed synergistic effect on modulating glucose metabolism via IRS-1/PI3K/Akt pathway. Mol Nutr Food Res. 2021; 65:e2100454.
39. Tasnuva ST, Qamar UA, Ghafoor K, Sahena F, Jahurul MH, Rukshana AH, Juliana MJ, Al-Juhaimi FY, Jalifah L, Jalal KCA, Ali ME, Zaidul ISM. ɑ-glucosidase inhibitors isolated from Mimosa pudica L. Nat Prod Res. 2019; 33:1495–1499.
40. Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res. 2012; 54:1773–1780.
41. Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001; 56:175-194.
42. Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980; 225:4758-4762.