Optimization of Flavonoid Extraction and Antioxidant Capacity from Acanthus ilicifolius Using Microwave-Assisted Extraction and I-Optimal Response Surface Methodology
Main Article Content
Abstract
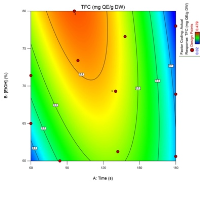
Acanthus ilicifolius is a medicinal plant from the Acanthaceae family commonly found in mangrove forests. Its extract contains antioxidant properties attributed to secondary metabolites, particularly flavonoids. Achieving optimal flavonoid content requires careful consideration of the extraction method. This study focuses on optimizing ethanol concentration and extraction time using I-optimal response surface methodology to maximize total flavonoid content (TFC) and antioxidant capacity in A. ilicifolius leaf extracts. The extraction was performed using varying ethanol concentrations and extraction duration. TFC was measured using a nanospectrophotometer, and antioxidant capacity was evaluated through CUPRAC (cupric ion reducing antioxidant capacity) and ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assays. Statistical analysis identified optimal conditions, with the highest TFC of 0.479 mg GAE/g DW obtained at 69.3% ethanol concentration and an extraction time of 130.2 seconds. Higher ethanol concentrations and longer extraction times generally enhanced flavonoid content and antioxidant activity. The highest antioxidant capacity, as measured by CUPRAC, was achieved at 76.6% ethanol and 138 seconds, yielding 14.149 µmol TE/g DW. The optimized extraction conditions resulted in predicted TFC and antioxidant capacities that closely matched experimental results, demonstrating the accuracy and reliability of the optimization model.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Velmani S, Perumal B, Santhosh C, Maruthupandian A. Phytochemical and traditional uses on Acanthus ilicifolius (L). J Adv Appl Sci Res. 2021;1(3):43–48.
Shameela KA, Johney J, Ragunathan R. Evaluation of antioxidant, antimicrobial, anticancer, and wound healing properties of leaf extracts of Acanthus ilicifolius L. Int J Curr Pharm Res. 2023;22–29.
Andriani D, Revianti S, Prananingrum W. Identification of compounds isolated from a methanolic extract of Acanthus ilicifolius leaves and evaluation of their antifungal and antioxidant activity. Biodiversitas. 2020;21(6):2521–2525.
Mtetwa MD, Qian L, Zhu H, Cui F, Zan X, Sun W, Wu D, Yang Y. Ultrasound-assisted extraction and antioxidant activity of polysaccharides from Acanthus ilicifolius. J Food Meas Charact. 2020;14(3):1223–1235.
Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med [Internet]. 2018 Apr 17;13(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5905184/
Fadhila SI, Hayati EK, Rafi M, Sabarudin A. Effect of ethanol-water concentration as extraction solvent on antioxidant activity of Acalypha indica. Al Kimiya: J Ilmu Kimia Terapan. 2023 Dec 31;10(2):133–142.
Das S, Mandal SC. Effect of process parameters of microwave-assisted extraction (MAE) on natural product yield from onion peel. Int J Pharm Sci Res. 2015;6(8):3260–3275.
Kataoka H. Pharmaceutical Analysis | Sample Preparation. In: Worsfold P, Poole C, Townshend A, Miró M, editors. Encyclopedia of Analytical Science. 3rd ed. Amsterdam: Elsevier; 2019. p. 231–255.
Reddy AVB, Moniruzzaman M, Madhavi V, Jaafar J. Recent improvements in the extraction, cleanup, and quantification of bioactive flavonoids. In: Rahman AU (Ed.). Studies in natural products chemistry. Volume 66. Amsterdam: Elsevier; 2020. p. 197–223. DOI: https://doi.org/10.1016/B978-0-12-817907-9.00008-8.
Batmomolin A, Ahsan A, Wiyasa IWA, Santoso S. Ethanolic extract of Moringa oleifera leaves improves inflammation, angiogenesis, and blood pressure in a rat model of preeclampsia. J Appl Pharm Sci. 2020;10(8):52–57.
George J, Edwards D, Pun S, Williams D. Evaluation of Antioxidant Capacity (ABTS and CUPRAC) and Total Phenolic Content (Folin-Ciocalteu) Assays of Selected Fruit, Vegetables, and Spices. Int J Food Sci. 2022;2022:1–18.
Irawan C, Putri ID, Sukiman M, Utami A, Ismail II, Putri RK, Lisandi A, Pratama AN. Antioxidant activity of DPPH, CUPRAC, and FRAP methods, as well as activity of alpha-glucosidase inhibiting enzymes from Tinospora crispa (L.) stem ultrasonic extract. Pharmacogn J. 2022;14(5):511–520.
Karthivashan G, Kura AU, Arulselvan P, Isa NM, Fakurazi S. The modulatory effect of Moringa oleifera leaf extract on endogenous antioxidant systems and inflammatory markers in an acetaminophen-induced nephrotoxic mice model. PeerJ. 2016;2016(7): e2127.
Kayahan S, Saloglu D. Optimization and kinetic modelling of microwave-assisted extraction of phenolic contents and antioxidants from Turkish artichoke. CyTA J Food. 2020;18(1):635–643.
Marliani N, Artika IM, Nurcholis W. Optimization extraction for total phenolic, flavonoid content, and antioxidant activity with different solvents and UPLC-MS/MS metabolite profiling of Justicia gendarussa Burm.f. CMU J Nat Sci. 2022;21(3): e2022046.
Nunes CR, Arantes MB, Pereira SMF, da Cruz LL, Passos MdS, Moraes LP, Vieira IJC, de Oliveira DB. Plants as sources of anti-inflammatory agents. Molecules. 2020;25(16):3726.
Van den Berg J, Kuipers S. The antibacterial action of Moringa oleifera: A systematic review. S Afr J Bot. 2022;151:224–233.
Susmayanti W, Kusumawati DE. The effect of extraction time and temperature on the antioxidant activity of Gnetum gnemon L. leaves. Indones J Med Pharm Sci. 2023;2(2):60-66.
Pratita ATK, Aisy NR, Wardani GA, Fathurohman M. Isolation and antioxidant activity using the ABTS method (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)) of superoxide dismutase compounds in Chlorella vulgaris microalgae. Proc Natl Semin Dissemin. 2022;177-184.
Noviyanty A, Salingkat CA, Syamsia. Effect of solvent ratio on the extraction from red dragon fruit (Hylocereus polyrhizus) peel. Kovalen. 2019;5(3):280-289.
Makkiyah FA, Pradana DLC, Putra RP, Nurcholis W. Optimization microwave-assisted extraction of Moringa oleifera leaves using response surface methodology focused on extracting phenolic and flavonoid with antioxidant activity. Trop J Nat Prod Res. 2024;8(6): 7474-7482.
Nurchoils W, Alfadzrin R, Izzati N, Arianti R, Vinnai BÁ, Sabri F, Kristóf E, Artika IM. Effects of methods and durations of extraction on total flavonoid and phenolic contents and antioxidant activity of Java cardamom (Amomum compactum Soland Ex Maton) fruit. Plants. 2022;11(17):2221.
Krisanta CS, Yusasrini NLA, Putra INK. Effect of ethanol concentration and extraction time on antioxidant activity of Buangit leaves extract (Cleome gynandra) using microwave- assisted extraction. Itepa J Ilmu Teknol Pangan. 2021;10(4):690-701.
Khairunnisa S, Hakim AR, Audina M. Comparison of total flavonoid content based on differences in ethanol solvent concentration from Centella asiatica (L.) Urban leaf extract. J Pharm Care Sci. 2022;3(1):121-131.
Artika IM, Kartiman R, Seno DSH, Ambarsari L, Putra RP, Nurcholis W. D-Optimal mixture design in sonication-maceration solvent extraction of total phenolic and antibacterial activity from Acanthus ilicifolius leaves. Trop J Nat Prod Res. 2023;7(12):5495-5500.
Islam MS, Islam MT, Washim MR, Haque AST, Haque MI, Islam HR, Rashid MH, Mahmud Y. Antioxidant potentials of Acanthus ilicifolius leaves from Southwest Coastal Region of Bangladesh. Food Chem Adv. 2024;5:100807.
Khalafyan AA, Temerdashev ZA, Yakuba YF, Guguchkina TI. Computer analysis of the sensory qualities of red wines as a method to optimize their blend formulation. Heliyon. 2019;5(5):e01602.