Antinutrients Properties and Antioxidant Activity of Some Nigerian Leafy Vegetable Plants
Main Article Content
Abstract
The leaves of red cocoyam (Colocasia esculenta), tropical nettle (Laportea aestuans L.), carrot (Daucus carota L.), cassava (Manihot esculenta, Crantz), and sesame (Sesamum indicum) are known for their numerous health benefits due to their high levels of phytochemicals like phenols and flavonoids. This study aims to evaluate the antinutrients properties and antioxidant activity of the leaves of these plants. The moisture and ash content were determined using the AOAC method. The total phenols, total flavonoids, vitamin A, total tannins, and total cyanogenic glycosides contents were determined following standard procedures. The antioxidant activity was assessed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2'-Azino-bis-(3-ethylbenzothiazoline-6- sulfonate) (ABTS+) radical scavenging assays. Among the plants, S. indicum leaves was found to have the highest total phenols and flavonoids contents with values of 825.95 mg GAE/100 g, and
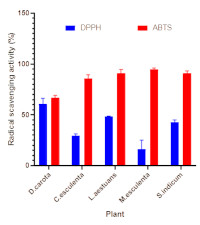
7.80 mg QE/100 g, respectively. The leaves of C. esculenta, D. carota, and M. esculenta had the highest vitamin A (7.63 mg/100 g), total tannins (112.27 mg/100 g), and total cynogenic glycosides (1792.65 mg/100 g) contents, respectively. The plants showed moderate to high antioxidant activity with D. carota exhibiting the highest DPPH radical scavenging activity (60.91%), while M. esculenta showed the highest ABTS radical scavenging activity (94.63%). The presence of antioxidant molecules and high radical scavenging activity suggest that these plants are potential sources of neutraceuticals, which could be harnessed in the management of oxidative stress-related diseases. However, the method of preparation that would reduce antinutrients, improve digestibility, and reduce toxicity of these plants should be adopted.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
1. Sá AGA, Moreno YMF, Carciofi BAM. Plant Proteins as high-quality Nutritional Source for Human Diet. Trends Food Sci Technol. 2020; 97:170– 184.doi.org/10.1016/j.tifs.2020.01.011
2. Goldstein R and Simon T. Toward a United Definition of Guild Structure for Feeding Ecology of North American Freshwater Fishes. In: Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities. Boca Raton: CRC Press; 2020. 123–202 p.
3. Utami YP, Yulianty P, Djabir YY, Alam G. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Etlingera elatior(Jack) R.M. Smith from North Luwu, Indonesia. Trop J Nat Prod Res. 2024; 8(1):5955- 5961.http://www.doi.org/10.26538/tjnpr/v8i1.34
4. Wang Y, Pruitt RN, Nürnberger T, Wang Y. Evasion of plant immunity by microbial pathogens. Nat Rev Microbiol. 2022; 20(8):449-464. doi.org/10.1038/s41579-022-00710-3
5. Kulczyński B and Gramza-Michałowska A. The Profile of Secondary Metabolites and Other Bioactive Compounds in Cucurbita pepo L. and Cucurbita moschata Pumpkin Cultivars. Molecules. 2019; 14(24/16):2945. doi.org/10.3390/molecules24162945
6. Rashmi HB and Negi PS. Phenolic Acids from vegetables: a Review on Processing Stability and Health Benefits Food Res Int. 2020; 136:109298. doi.org/10.1016/j.foodres.2020.109298
7. Sarkar T, Salauddin M, Roy A, Sharma N, Sharma A, Yadav S, Jha V, Rebezov M, Khayrullin M, Thiruvengadam M, Chung IM, Shariati MA, Simal-Gandara J.. Minor Tropical Fruits as a Potential Source of Bioactive and Functional Foods. Crit Rev Food Sci Nutr. 2022; 63(23):6491–6535. doi.org/10.1080/10408398.2022.2033953
8. Tran N, Pham B, Le L. Bioactive Compounds in Anti- Diabetic Plants: from Herbal Medicine to Modern Drug Discovery. Biol.2020;9(9):252. doi.org/10.3390/biology9090252
9. Ghildiyal R, Prakash V, Chaudhary VK, Gupta V, Gabrani R. Phytochemicals as Antiviral Agents: Recent Updates. In: Plant-derived Bioactives. Springer Singapore; 2020. p. 279– 295.doi.org/10.1007/978-981-15-1761-7_12
10. Martel J, Ojcius DM, Ko YF, Ke PY, Wu CY, Peng HH, Young JD. Hormetic Effects of Phytochemicals on Health and Longevity. Trends Endocrinol Metab. 2019; 30(6):335– 346. doi.org/10.1016/j.tem.2019.04.001
11. García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid Med Cell Longev. 2020; 2020:1–16. doi.org/10.1155/2020/2082145
12. Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit Rev Food Sci Nutr. 2015; 56(3):419–444. doi.org/10.1080/10408398.2013.767221
13. Leisner CP. Review: Climate Change Impacts on Food security- Focus on Perennial Cropping Systems and Nutritional Value. Plant Sci. 2020; 293:110412. doi.org/10.1016/j.plantsci.2020.110412
14. Horwitz W and Latimer G. Official Methods of Analysis of the Association of Analytical Chemists. 1970.
15. McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chem. 2001; 73(1):73–84. doi.org/10.1016/S0308- 8146(00)00288-0
16. Chang CC, Yang MH, Wen HM, Chern JC. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J Food Drug Anal. 2002; 10(3):Article 3.
17. Pearson D. The Chemical Analysis of Food. London, UK: J.&A. Churchill; 1970.
18. Awe I and Sodipo O. Purification of Saponins of Root of Bhlighid sapida Koenig-Holl. Nig J Biochem Mol Biol. 2001; 16:201–204.
19. Onwuka SK and Olopade JO. Some aspects of the clinical anatomy of the mandibular and maxillofacial Regions of the West African dwarf goat in Nigeria. Int J Morphol. 2005; 23(1):33-36.
20. Gyamfi MA, Yonamine M, Aniya Y. Free-radical Scavenging Action of Medicinal Herbs from Ghana. Gen Pharmacol Vasc Syst. 1999; 32(6):661–667. doi.org/10.1016/S0306-3623(98)00238-9.
21. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice- Evans C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol Med. 1999; 26(9/10):1231–1237. doi.org/10.1016/S0891- 5849(98)00315-3.
22. Ma Y, Yi J, Jin X, Li X, Feng S, Bi J. Freeze-Drying of Fruits and Vegetables in Food Industry: Effects on Phytochemicals and Bioactive Properties Attributes - a Comprehensive Review. Food Rev Int. 2022; 39(9):6611–6629. doi.org/10.1080/87559129.2022.2122992.
23. Keshav A, Sharma A, Mazumdar B. Phytochemical Analysis and Antioxidant Activity of Colocasia esculenta (L.) Leaves. Int J Chem Mol Eng. 2019; 13(1):20–23. ISNI:0000000091950263
24. Teng H and Chen L. Polyphenols and bioavailability: an Update. Crit Rev Food Sci Nutr. 2019; 59(13):2040–2051. doi.org/10.1080/10408398.2018.1437023
25. Rodríguez-García C, Sánchez-Quesada C, Gaforio JJ. Dietary Flavonoids as Cancer Chemopreventive Agents: an Updated Review of Human Studies. Antioxidants. 2019; 8(5):137. doi.org/10.3390/antiox8050137
26. Cosme P, Rodríguez AB, Espino J, Garrido M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants. 2020; 9(12):1263. doi.org/10.3390/antiox9121263
27. Kim S, Yang HY, Lee HJ, Ju J. In Vitro Antioxidant and Anti-Colon Cancer Activities of Sesamum indicum L. Leaf Extract and Its Major Component, Pedaliin. Foods (Basel, Switzerland). 2021; 10(6):1216. doi.org/10.3390/foods10061216
28. Hridhya KV, Kulandhaivel M, Srinivasan P, Rajalakshmi M. Identification of Bioactive Compounds in Sesamum alatum Thonn by Qualitative Phytochemical Analysis and GC-MS. Biochem Cell Arch. 2023; 23(1):329–334.
29. Nwanagba N, Ukam A, Okoli J, Okparauka I, Ubadire-Agua C. Evaluation of Functional and Chemical Properties of Composite Flour Made from Unripe Plantain (Musa paradisiaca) and Cocoyam (Colocasia esculenta) and Assessment of Consumers’ Acceptability of Amala. Int J Home Econ Hosp Allied Res. 2022; 1(2):246–256.
30. Huang Z, Liu Y, Qi G, Brand D, Zheng S. Role of Vitamin A in the Immune System. J Clin Med. 2018; 7(9):258. doi.org/10.3390/jcm7090258.
31. Ayoub Z and Mehta A. Medicinal Plants as Potential Sources of Antioxidants Agent: a Review. Asian J Pharm Clin Res. 2018; 11(6):50–56.
doi.org/10.22159/ajpcr.2018.v11i6.24725
32. Larramendy M and Soloneski S. Toxicology: New Aspects to This Scientific Conundrum. Google Books. BoD – Books on Demand; 2016.
33. Koubala B, Laya A, Massaï H, Kouninki H, Nukenine E. Physico-chemical Characterization Leaves from Five Genotypes of Cassava (Manihot esculenta Crantz) Consumed in the Far North Region (Cameroon). Am J Food Sci Technol. 2015; 3(2):40–47. doi:10.12691/ajfst-3-2-3
34. Cressey P and Reeve J. Metabolism of Cyanogenic glycosides: a Review. Food Chem Toxicol. 2019; 125:225– 232. doi.org/10.1016/j.fct.2019.01.002
35. Setyorini D and Antarlina SS. Secondary Metabolites in Sorghum and Its Characteristics. Food Sci Technol. 2022; 42:e49822. doi.org/10.1590/fst.49822
36. Samtiya M, Aluko RE, Dhewa T. Plant Food anti-nutritional Factors and Their Reduction strategies: an Overview. Food Prod Proc Nutr. 2020; 2:1-14. doi.org/10.1186/s43014-020- 0020-5.
37. McLean E, Klemm R, Subramaniam H, Greig A. Refocusing Vitamin A Supplementation Programmes to Reach the Most Vulnerable. BMJ Global Health. 2020; 5(7):e001997. doi.org/10.1136/bmjgh-2019-001997
38. Danet AF. Recent Advances in Antioxidant Capacity Assays [Internet]. www.intechopen.com. IntechOpen; 2021. Available from:
https://www.intechopen.com/chapters/75789
39. Masek A, Chrzescijanska E, Latos M, Kosmalska A. Electrochemical and Spectrophotometric Characterization of the Propolis Antioxidants Properties. Int J Electrochem Sci. 2019; 14(2):1231–1247. doi.org/10.20964/2019.02.66