Physical and Chemical Compatibility Testing of Intravenous Phenytoin Preparations In 0.9% Normal Saline Solution
Main Article Content
Abstract
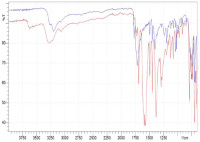
Intravenous (IV) phenytoin sodium preparations have high pH values (>12) and present compatibility problems with admixtures. Hence, it is necessary to dissolve it in normal (0.9% w/v) saline to adjust the pH to avoid incompatibilities with other medicines, stability, and prevent phlebitis. This study aims to determine the physical and chemical incompatibility of IV phenytoin preparations in normal saline over a certain period. Physical testing of IV phenytoin in normal saline, including organoleptic particle size and pH measurements at 0, 3, and 6 hours. Chemical stability testing was carried out by measuring the concentration of the preparation for 8 hours using a UV spectrophotometer, testing the functional groups of phenytoin IV precipitates in normal saline using FTIR and molecular weight using GC-MS. The results of testing the concentration of phenytoin samples A, B, and C showed instability in the preparations. In the FTIR test, the sample showed absorption bands at 1752 cm-1 for the amide (C=O) functional group, 744 cm-1 for the phenyl C-H group, and 1286 cm-1 for the C-N group. Results of the physical characteristics test showed no increase in pH of more than 1 unit from 0, 3, and 6 hours for the three samples tested, but there was an increase in turbidity of the preparation from visual observation. The MS analysis showed that the pure phenytoin and the precipitated preparation have the same molecular weight of 252.1 m/z.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Pradipta IS, Pratama D, Khatami H, Sanggelorang J. Potential drug-drug interaction in the intensive care unit: an observational study at a hospital in Bandung. Indones J Clin Pharm. 2022;11:41–50.
Vendrell AM, Teixidor S, Padro JS, Compoy S, Villanueva MH. Intravenous lacosamide and phenytoin for the treatment of acute exacerbations of trigeminal neuralgia: A retrospective analysis of 144 cases. J fur Neurol Neurochir und Psychiatr. 2022;23:1031–1038.
Adeleye OA, Ayorinde JO, Bakre LG, Bamiro OA. Pharmaceutical stability of brands of paracetamol tablets under different environmental conditions. Trop J Nat Prod Res. 2019;3:149–154.
Lee KS, Lee BL. Efficacy of single dose of phenytoin/fosphenytoin in benign convulsions with mild gastroenteritis. Turk J Pediatr. 2023;65:109–117.
Lacy FC, Armstrong LL, Goldman PM, Lance LL. Drug Information Handbook, ed. 17th. American Pharmacists Association, North American. 2009.
Zhu LL, Wang YH, Zhou Q. Progress in research on the mechanisms and interventions of phlebitis from the perspective of vascular endothelial cell and signalling pathway. J Inflamm Res. 2023;16:6469–6481.
Rahman Z, Dharani S, Barakh Ali SF, Nutan MTH, Khan MA. Effects of diluents on physical and chemical stability of phenytoin and phenytoin sodium. AAPS J PharmSciTech. 2020;21:1–14.
Maharani L, Astuti A, Achmad A. Parenteral admixture compatibility in neurosurgery ward in Prof. Dr. Margono Soekarjo regional public hospital. Indones J Clin Pharm. 2014;3:1–9.
Vallée M, Barthélémy I, Friciu M, Pelletier É, Forest JM, Benoit F, et al. Compatibility of lactated ringer's injection with 94 selected intravenous drugs during simulated y-site administration. J Hosp Pharm. 2021 Aug 1;56:228–234.
Indu T, Basutkar R. Hypersensitivity reaction associated with phenytoin. J Basic Clin Pharm. 2015;6:119.
Masaki Y, Tanaka M, Nishikawa T. Physicochemical compatibility of propofol-lidocaine mixture. J Anesth Analg. 2003;97:1646–1651.
Menon VB, Kurian J, Undela K, Ramesh M, Gowdappa HB. Phenytoin toxicity: A case report. J Young Pharm. 2015;7:272–275.
Salamah AU, Rahmawati F, Kurniawati F. Potential incompatibility problem of intravenous drugs' administration among intensive care unit (ICU) patients at PKU Muhammadiyah Yogyakarta Hospital. J Manag Pharm Pract. 2019;9:238–242.
Nokhodchi A, Bolourtchian N, Dinarvand R. Crystal modification of phenytoin using different solvents and crystallisation conditions. Int J Pharm. 2003;250:85–97.
Somia Gul and Asra Hameed. UV spectroscopic method for determination of phenytoin in bulk and injection forms. Chem Int. 2018;4:177–182.
Deodhar M, Sable P, Bhosale A, Juvale K, Dumbare R, Sakpal P. Synthesis and evaluation of phenytoin derivatives as anticonvulsant agents. Turkish J Chem. 2009;33:367–373.
Khan J, Khan A, Tahirabashir. Synthesis and characterisation of phenytoin drug and alpha benzilmonoxime from BEA711. Int J Med Work. 2015;1:5–8.
Perez M, Décaudin B, Foinard A, Barthélémy C, Debaene B, Lebuffe G, et al. Compatibility of medications during multi-infusion therapy: A controlled in vitro study on a multilumen infusion device. J Anaesth Crit Care Pain Med. 2015;34:83–88.
S. Sriram, Aishwarya S, Kumar AA, Moithu A, Sebastian A. Intravenous drug incompatibility in intensive care units - A comprehensive review. J Innov Pharm Pharmacother. 2018;6:55–60.
Avery LM, Chen IH, Reyes S, Nicolau DP, Kuti JL. Assessment of the physical compatibility of eravacycline and common parenteral drugs during simulated y-site administration. J Clin Ther. 2019;41:2162–2170.
Dettlaff K, Dominiak K, Klimaszewska M, Gostyńska A. Physical compatibility of ibuprofen and selected parenteral drugs during simulated y-site administration. J Acta Pol Pharm - Drug Res. 2023;80:255–267.
Safari J, Naeimi H, Ghanbari MM, Sabzi Fini O. Preparation of phenytoin derivatives under solvent-free conditions using microwave irradiation. Russ J Org Chem. 2009;45:477–479.
Patocka J, Wu Q, Nepovimova E, Kuca K. Phenytoin – An anti-seizure drug: Overview of its chemistry, pharmacology and toxicology. J Food Chem Toxicol. 2020;142:111393.
Ballesteros-Peña S, Fernández-Aedo I, Vallejo-De la Hoz G, Tønnesen J, Miguelez C. Identification of potentially irritating intravenous medications. J Enfermería Intensiva. 2022;33:132–140.
Surakitbanharn Y, Simamora P, Ward GH, H. Yalkowsky S. Precipitation of pH solubilised phenytoin. Int J Pharm. 1994;109:27–33.
Takahashi M, Lee YJ, Kanayama T, Kondo Y, Nishio K, Mukai K, Haba
M, Hosokawa M. Design, synthesis, and biological evaluation of water-soluble phenytoin prodrugs considering the substrate recognition ability of human carboxylesterase 1. Eur J Pharm Sci. 2020;152:105455.
Agarwal SP, Blake MI. Determination of the pKa' value for 5,5‐diphenylhydantoin. J Pharm Sci. 1968;57:1434–1435.
Alvarez-Núñez FA, Yalkowsky SH. Buffer capacity and precipitation control of pH solubilised phenytoin formulations. Int J Pharm. 1999;185:45–49.
Guerrab W, Lgaz H, Kansiz S, Mague JT, Dege N, Ansar M, et al. Synthesis of a novel phenytoin derivative: Crystal structure, Hirshfeld surface analysis, and DFT calculations. J Mol Struct. 2020;1205.
Jansod S, Afshar MG, Crespo GA, Bakker E. Phenytoin speciation with potentiometric and chronopotentiometric ion-selective membrane electrodes. J Biosens Bioelectron. 2016;79:114–120.
Panthong S, Sakpakdeejaroen I, Kuropakornpong P, Jaicharoensub J, Itharat A. Antibacterial activity and stability evaluation of "Apo-taat" remedy extract for inhibiting diarrhoea-causing bacteria. Trop J Nat Prod Res. 2020;4:1101–1107.
Gupta D, Bhatia D, Dave V, Sutariya V, Gupta SV. Salts of therapeutic agents: Chemical, physicochemical, and biological considerations. J Mol. 2018;23:1–15.
Schwartz PA, Rhodes CT, Cooper JW. Solubility and ionisation characteristics of phenytoin. J Pharm Sci. 1977;66:994–997.
Thakker D, Raval A, Patel I, Walia R. N-Acetylcysteine for Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. J Obstet Gynecol Int. 2015;2015:1–13.
Stella VJ. A case for prodrugs: Fosphenytoin. J Adv Drug Deliv Rev. 1996;19(2):311–330.