Molecular Docking of Phytochemicals from Vernonia amygdalina and Nauclea latifolia as Potential Inhibitors of Drug Targets in Ascaris lumbricoides
Main Article Content
Abstract
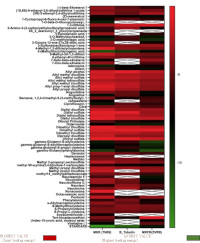
Ascariasis is a parasitic infection caused by the intestinal roundworm Ascaris lumbricoides. It is one of the soil-transmitted helminths (STHs), also known as geohelminths. Human and animal ascariasis constitutes one of the most important global public health challenges with worldwide distribution, with the majority of cases found in the tropics, such as Nigeria, India, and China. The study was performed in silico on secondary metabolites isolated from medicinal plants Vernonia amygdalina and Nauclea latifolia based on an ethnobotanical survey made in Nigeria. Maestro 9.3 docking suite was used in protein preparation, ligand preparation, receptor grid formation, and ligand docking against different A. lumbricoides specific proteins (3VR9, 7N09, Beta-tubulin) that have been documented as potential and conventional drug targets. One hundred and eighty-two phyto-compounds of N. latifolia and Bitter leaf were obtained from examination of already published works of literature Journals and used in this study for the generation of library of compounds respectively. The result showed the docking score of the two plants to each of the specific proteins and also the ligand interactions of these phytochemicals to the specific protein. The investigations of this current project through molecular docking studies have shown that phytochemicals from the medicinal plants; V. amygdalina and N. latifolia can serve as potential inhibitors and/or lead compounds for the development of novel inhibitors of these targets for the treatment of ascariasis. It also provides the molecular basis of their anti-helminthic activities, possibly through the inhibition of metabolic processes in the mitochondria.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Kochifa, A.M., Mohammed, M.T. and Sani, Y.M. Prevalence of Ascariasis in Selected Communities of Adamawa Northern Senatorial District. Biomed Sci. 2021, 7(2), 60-63
Nigerian Center for Disease Control (NCDC), (2017). Soil-transmitted Helminths. Retrieved from www.ncdc.gov.ng/diseases. Accessed on 24th July 2024
Loukas, A., Maizels, R.M., Hotez, P.J. The yin and yang of human soil-transmitted helminth infections. Int J Parasitol. 2021;51(13–14):1243–1253.
Bishop, H.G. and Yohanna, A.Z. Contamination of vegetables with geohelminths: prevalence, intensity, and roles of hygiene practices in Samaru-Zaria, Nigeria. Int. J. Acad Appl Res. 2018; 2(7), 8-13
Okoh, M.E., Nyinoh, I.W., Utume, L.N. and Terzunwe, T.T. Prevalence of Ascariasis among Children in Makurdi, Benue State, Nigeria. J. Adv. Microbiol. 2021, 21, 69-73.
Taiwo, O.T., Sam-Wobo, S.O. and Taiwo, A.M. Spatial distribution of helminth infections in Nigeria and the need for attitudinal and behavioral changes in the water, Sanitation, and Hygiene Interventions. Ife J Sci. 2016; 18(4), 1-10.
"Parasites - Ascariasis" (https://www.cdc.gov/parasites/ascariasis/). U.S. Centers for Disease Control and Prevention. 13 June 2023. Retrieved 24 July 2024.
Azamu, I.G., Oti, V.B., Oti, I.B., Philip, A.A., and Anizoba E. Ascaris lumbricoides infection using microscopy and IgG4 detection techniques in school children population in Central Nigeria., J Infect Dis Treat 2018; 4(1), 1-5.
Parvatham, K., Veerakumari, L. and Shoba, G. Molecular docking studies of acetate-succinate CoA-transferase of Ascaris lumbricoides with a few phytochemicals and anti-helmintics. J. Computat. Meth. Mol. Design. 2015; 5(4), 1-10
Yuan, H., Ma, Q., Ye, L. and Piao, G. The traditional medicine and modern medicine from natural products, Molecules, 2016; 21(5), 559.
Adenike, A.O., Samuel, A. and Christian, O.O. Preliminary investigation of Nauclea latifolia ripe fruits for antioxidant and antidiabetic activities. J. Appl. Nat. Sci 2019; 11(3), 718-723
Singla, S. and Kaur, S. Anthelmintic lead compounds and their targets for drug development. J. Ayu. Herb. Med. 2021; 7(4): 265-275
Bauri, R.K., Tigga, M.N. and Kullu, S.S. A review on the use of medicinal plants to control parasites. Indian J Nat Prod Resour 6(4) 268-277, 2015; 6(4), 268-277
Ugbogu, E.A., Emmanuel, O., Dike, E.D., Agi, G.O., Ugbogu, O.C., Ibe, C., Iweala, E.J. The Phytochemistry, Ethnobotanical, and Pharmacological Potentials of the Medicinal Plant-Vernonia amygdalina L. (Bitter leaf). Clinic Complement Med Pharmacol. 2021; 1(1), 100006.
Ijeh, I.I., Ejike, C.E.C.C. "Current perspectives on the medicinal potential of Vernonia amygdalina Del". J Med Plant Res. 2011: 5 (7): 1051–1061
Tekou, F.A., Kuate, D., Nguekouo, P.T., Woumbo, C.Y., Oben, J.E. Effect of cooking treatments on the phytochemical composition and antidiabetic potential of Vernonia amygdalina. Food Sci Nutr. 2018; 6(6), 1684–1691
Gbonhinbor, J., Abah, A.E. and Awi-Waadu, G. Prevalence of Intestinal Parasitic Infection and Associated Risk Factors Among Primary School-Aged Children (5 - 15 years) in Southern Nigeria. Int J Infect. 2022; 9(3), e123721
Danquah, C.A., Koffuor, G.A., Annan, K. and Ketor, E.C. The Anthelmintic Activity of Vernonia amygdalina (Asteraceae) and Alstoniaboonei De Wild (Apocynaceae). J Med Biom Sci, 2012; 1(1), 21-27.
Siamba, D.N., Okitoi, L.O., Watai, M.K., Wachira, A.M., Lukibisi, F.B. and Mukisira, E.A. Efficacy of Tephrosia vogelli and Vernonia amygdalina as anthelmintics against Ascaridiagalliin indigenous chicken. Livestock Res Rural Dev. 2007; 176.
Alawa, C.B.I., Adamu, A.M., Gefu, J.O., Ajanusi, O.J., Abdu, P.A. and Chiezey, N.P. In vivo efficacy of Vernonia amygdalina (Compositae) against natural helminth infection in Bunaji (Bos indicus) calves. Pak Vet J. 2010; 30(4), 215-218.
Release, S. 2018-2: Maestro, version 9.3. Schrödinger, LLC, New York, NY, 2018. https://www.schrodinger.com/maestro
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S. and Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009; 30(16), 2785-2791.
Dong, H., & Chang, M. C. Y. Structural Basis for Branched Substrate Selectivity in a Ketoreductase from Ascaris suum. ACS Catalysis, 2021; 11(14), 8948–8955
Parvatham, K., Veerakumari, L. and Shoba, G. Molecular docking studies of acetate-succinate CoA-transferase of Ascaris lumbricoides with a few phytochemicals and anti-helmintics J. Computat. Meth. Mol. Design, 2015; 5(4), 1-10
Wang J. Fast Identification of Possible Drug Treatment of Coronavirus Disease-19 (COVID-19) through Computational Drug Repurposing Study. J Chem Inf Model. 2020; 60(6):3277-3286.
Haudecoeura R., Peuchmaura M., Pérèsa B., Romec M., Taïwee G. S., Boumendjela A., and Boucherlea, B. Traditional uses, phytochemistry and pharmacological properties of African Nauclea species: A review. J. Ethnopharmaco, 2018; 212, 106-136.
Binarová, P., & Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells, 2019; 8(10).
Sugianto, R., Sukarno, V., Sudarmaja, M. and Swastika, K. Water source as the main risk factor of soil-transmitted helminth infection on primary school students in Antiga Village, Bali. Asian J Pharm Clin Res. 2019; 12, 119-121
Kadiri, O. and Olawoye, B. Vernonia amygdalina: An Underutilized vegetables with Nutraceutical potentials- A review. Turkish JAF Sci.Tech 2016; 4(9), 763-768.