Effect of Phoenix reclinata Jacq Methanol Leaf Extract and Fractions on Plasmodium berghei Infected Mice
Main Article Content
Abstract
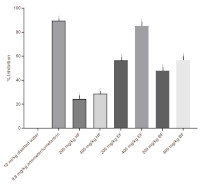
Malaria treatment is burdened by many challenges including growing resistance to currently used antimalarial drugs. Thus, the continuous search for safe and effective remedies is necessary. This study investigated the antiplasmodial activities of the methanol extract (ME) of Phoenix reclinata and its n-hexane (HF), ethyl acetate (EF), and butanol (BF) fractions. Phytochemistry of the extract and fractions was conducted. The effect of methanol extract (100, 200, and 400 mg/kg) and its solvent fractions (200 and 400 mg/kg) on parasitaemia levels was evaluated using curative, suppressive, and prophylactic anti-plasmodial models. The antipyretic effect of its solvent fractions was also determined. In the curative model, ME exhibited dose-dependent chemosuppression of 18.4%, 46.4%, and 79.6% at 100, 200, and 400 mg/kg. At 200 and 400 mg/kg, HF produced 24.5% and 28.9%, EF produced 56.8% and 85.3%, and BF produced 48% and 56.8%. In the Suppressive model, ME exhibited a dose-dependent chemosuppression of 19.08%, 45.23%, and 80% by 100, 200, and 400 mg/kg, HF exhibited 15.55% and 28.89%, EF exhibited 61.32% and 81.94%, BF exhibited 34.06% and 43.08% for 200 and 400 mg/kg. In the prophylactic model, ME exhibited a dose-dependent chemosuppression of 8.71 %, 15.59%, and 17.48 % by 100, 200, and 400 mg/kg. HF exhibited 2.66% and 8.6% for 200 and 400 mg/kg. BF exhibited anti-plasmodial suppression of 15.73% and 21.68% for 200 and 400 mg/kg. EF exhibited the highest chemosuppression in all the models.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Ali BH, Mohammed I, Muhammed MA, Mohammed SY, Garba D, Busola OA, Khadijah II, Manager MM. Evaluation of In vivo Antiplasmodial Activity of the Methanol Root Bark Extract and Fractions of Bombax costatum(Bombacacea) in Plasmodium berghei-Infected Mice.Trop J Nat Prod Res. 2022; 6(6):926-930.DOI: 10.26538/tjnpr/v6i6.18
Biswajit B. A short review of laboratory diagnosis of malaria. Int J Res Pharm Biomed Sci. 2013; 2(5):177-181
Uzoka FME, Akwaowo C, Nwafor‑Okoli C, Ekpin V, Nwokoro C, El Hussein M, Osuji J, Aladi F, Akinnuwesi F, Akpelishi TF. Risk factors for some tropical diseases in an African country. BMC Public Health. 2021; 21:2261 DOI:.1186/s12889-021-12286-3
World Malaria Report 2023; https://www.who.int/news-room/fact-sheets/detail/malaria
Lacey MS. Walter TW. Plasmodium vivax, StatPearls Publishing. National Center for Biotechnology Information (NCBI), U.S. Nat Lib Med. 2021; 8600 Rockville Pike, Bethesda MD, 20894 USA.
Singh B, KimSung L, Matusop A. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004; 363 (9414): 1017–1024.
Ekechukwu PA. Prevalence rate of Plasmodium species among the inhabitants in Owerri Metropolis. B.Sc. Project Report submitted to the Department of Medical Laboratory Sciences Imo State University Owerri, Nigeria .2005
Chinwuba P, Akah PA, Ilodigwe EE. In vivo antiplasmodial activity of the ethanol stem extract and fractions of Citrus sinensis in mice. Merit Res. J. Med. Med. Sci. 2015; 3: 140 - 146.
Barrow SC. Phoenix reclinata. Kew Bulletin 1998;53 (3), 513-575
Ruffo CK, Birnie A, Tengnas B. Edible Wild Plants of Tanzania.
Regional Land Management Unit; 2002; Nairobi.ISBN 9966-896-60-0
Veronica R. Phoenix reclinata in The Shell Field Guide to the Common Trees of the Okavango Delta and Moremi Game Reserve. Gaborone, Botswana: Shell Oil Botswana,1992.
Chinwuba P, Achunike PA, Ugorji P, Nworu CS. Anti-Inflammatory, and Antipyretic Activity of Methanol Leaf Extract of Phoenix reclinata, Jacq (Aracaceae). Trend Nat Prod Res 2024; 5(1). 44-51. DOI:10.61594/tnpr. v5i1.2024.104
Charles DK, Cissy BN, Kokas I, Andrew T, Gabriel T, John K. Nephroprotective Effect of Phoenix reclinata Total Crude Root Extract on Tenofovir Induced Kidney Damage in Wistar Albino Rats. J. Pharm Res Int. 2017; 17(6): 1-10. DOI: 10.9734/JPRI/2017/34247
Hortense BS, Yolande DD, Valery NKP, Betty FM, Maxwell BAG, Patrice KL. Physicochemical and Nutritional Properties of Date (Phoenix reclinata) from Dabou (Côte d’Ivoire). Asian J. Crop. Soil Plan. Nutri. 2019; 5(2): 1-8.
Oljira Kenea OK, Habte Tekie HT. Ethnobotanical survey of plants traditionally used for malaria prevention and treatment in selected resettlement and indigenous villages in Sasiga district, Western Ethiopia. J Biol Agric Healthc.2015;5(11), 1-9.
Ogbeide OK, Dickson VO, Jebba RD, Owhiroro DA, Olaoluwa MO, Imieje VO, Erharuyi O, Owolabi BJ, Fasinu P, Falodun A. Antiplasmodialand Acute Toxicity Studies of Fractions and Cassane-Type Diterpenoids from the Stem Bark of Caesalpinia pulcherrima (L.) Sw. Trop J Nat Prod Res. 2018; 2(4):179-184. Doi: 10.26538/tjnpr/v2i4.5
Odetola, A. and Bassir, O. Evaluation of Antimalarial properties of some Nigerian Medicinal Plants. In: Sofowora, A. editor, Proceeding of African Bioscience Network, Fed. Min. of Science and Tech., Nigeria Society of Pharmacognosy and Drug Research. And Production Unit, University of Ife. 1980
Trease GE, Evans WC. Pharmacognosy. 15th Ed. London: Saunders Publishers 2002; pp. 42–393
Ryley JF, Peters W. The antimalarial activity of some quinolone esters. Ann. trop. med. Parasitol. 1970; 84:209-222
Knight DJ, Peters W. The antimalarial action of N-benzyloxy dihydro triazines. The action of cycloguanil (BRL50216) against rodent malaria and studies on its mode of action. Ann. trop. med. Parasitol. 1980; 74: pp. 393–404.
Peters W. Drug resistance in plasmodium berghei. Vinka and Lips. Exp. Parasitol. 1965; 17: 80-89
Sini KR, Sinha BN, Karpakavalli M, Sangeetha PT. Analgesic and antipyretic activity of Cassia occidentalis Linn. Ann Biol Res. 2011; 2 (1):195-200
Okaiyeto K, Hoppe H, Okoh AI. Plant-Based Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Salvia officinalis: Characterization and its Antiplasmodial. Activity. J Cluster Sci. 2021;32:101–109. DOI:10.1007/s10876-020-01766-y.
Dinarello CA. Blocking interleukin-1 and tumour necrosis factor in disease. Euro Cytok Net.1997; 8: 294-296.
Mamadou W, Singou K, Cheickna C, Nouhoum D, Laura K, Lamine B. Antibacterial and Antiplasmodial Activities of Tannins Extracted from Zizyphus mauritiana in Mali. Int J of Biochem Res & Rev 2018; 24(2):1-8. DOI: 10.9734/IJBCRR/2018/45335
Weathers PJ, Towler M, Hassanali A, Lutgen P, Engeu PO. Dried-leaf Artemisia annua: a practical malaria therapeutic for developing countries? World J of Pharmac.2014; 3:39–55.
Mueller MS, Karhagomba IB, Hirt HM, Wemakor E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. J. Ethnopharm.2000;73 487–493. DOI:10.1016/S0378-8741(00)00289-0
Czechowski T, Mauro AR, Mufuliat TF, Maria VV, Tony RL., Thilo W, Deborah AR, David H, Paul H, Ian AG. Flavonoid Versus Artemisinin Anti-malarial Activity in Artemisia annua Whole-Leaf Extracts. Frontiers in Plant Sci. 2019; 10: 984. DOI: 10.3389/fpls.2019.00984
Kumar V, Abbas AK, Fausto N. Acute and chronic inflammation. In:
Kumar V, Abbas AK, Fausto N, ed., Robbins and Cotran Pathologic Basis of Disease, 7th ed. Philadelphia, Elsevier Saunders, 2005. 47–86 p.