Assessment of the Antiplasmodial and Haematoprotective Potential of a Polyherbal Extract of Azadirachta indica, Mangifera indica, and Persea americana Leaves in Mice Infected with Plasmodium berghei
Main Article Content
Abstract
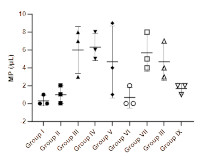
The polyherbal extract obtained from the leaves of Azadirachta indica, Mangifera indica, and Persea americana is traditionally utilized by the Esan people of Edo State, Nigeria, to treat uncomplicated malaria. This study assessed the antiplasmodial and haematoprotective efficacy of the polyherbal extract via in vivo tests utilizing chloroquine-sensitive mice infected with Plasmodium berghei, focusing on parasite count and its effects on haematological markers. The animals were allocated to treatment groups receiving 200, 400, and 800 mg/kg of fresh and heated polyherbal extracts, respectively, with chloroquine as the reference drug. The malaria parasite count was conducted via Field's staining method, while the whole blood count was performed with an auto-hematology analyzer. The results of the study revealed a dose-dependent antiparasitic effect of the polyherbal extract, with the 800 mg/kg dosage reducing red blood cell count. Although the malaria parasite count was not significantly different among the treated groups, those receiving 800 mg/kg daily of both heated and fresh extracts showed parasite levels comparable to the normal and chloroquine-treated control groups. No significant differences were observed in the other red blood cell parameters or white blood cell counts across the groups, including the chloroquine-treated group. The group treated with the heated extract at 800 mg/kg showed the lowest average white blood cell count. This study offers significant insights into the therapeutic potential of the polyherbal extract. Additional research is required to clarify the mechanisms behind the reported effects and to investigate the therapeutic benefits of adequately controlling malaria symptoms.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Oladeji OS, Oluyori AP, Bankole DT, Afolabi TY. Natural products as sources of antimalarial drugs: Ethnobotanical and ethnopharmacological studies. Scientifica. 2020; 2020: 1–22. Doi: 10.1155/2020/7076139
]Faloye KO, Adesida SA, Oguntimehin SA, Adewole AH, Omoyeni OB, Fajobi SJ, Ugwo JP, Asiyanbola ID, Bamimore VO, Fakola EG, Oladiran OJ, Spiteller M. LC-MS analysis, computational investigation, and antimalarial studies of Azadirachta indica fruit. Bioinform. Biol. Insights. 2023; 17: 1–9. Doi: 10.1177/11779322231154966
Shibeshi MA, Kifle ZD, Atnafie SA. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect. Drug Resist. 2020; 13: 4047–4060. Doi: 10.2147/IDR.S279433
Erhirhie EO, Ikegbune C, Okeke AI, Onwuzuligbo CC, Madubuogwu NU, Chukwudulue UM, Okonkwo OB. Antimalarial herbal drugs: a review of their interactions with conventional antimalarial drugs. Clin. Phytoscience. 2021; 7:1–10. Doi: 10.1186/s40816-020-00242-4
Angupale JR, Tusiimire J, Ngwuluka NC. A review of efficacy and safety of Ugandan anti-malarial plants with application of RITAM score. Malar. J. 2023; 22:1–19. Doi: 10.1186/s12936-023-04486-6
Tudu CK, Bandyopadhyay A, Kumar M, Radha, Das T, Nandy S, Ghorai M, Gopalakrishnan AV, Proćków J, Dey A. Unravelling the pharmacological properties of cryptolepine and its derivatives: a mini-review insight. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023; 396, 229–238. Doi: 10.1007/s00210-022-02302-7
Tabuti JRS, Obakiro SB, Nabatanzi A, Anywar G, Nambejja C, Mutyaba MR, Omara T, Waako P. Medicinal plants used for treatment of malaria by indigenous communities of Tororo District, Eastern Uganda. Trop. Med. Health. 2023; 51:1–15. Doi: 10.1186/s41182-023-00526-8
Khairani S, Fauziah N, Wiraswati HL, Panigoro R, Setyowati EY, Berbudi A. Oral Administration of Piperine as Curative and Prophylaxis Reduces Parasitaemia in Plasmodium berghei ANKA-Infected Mice. J. Trop. Med. 2022; 2022:1–11. Doi: 10.1155/2022/5721449
Jamil SNH, Ali AH, Feroz SR, Lam SD, Agustar HK, Mohd Abd Razak MR, Latip J. Curcumin and its derivatives as potential antimalarial and anti-inflammatory agents: A review on structure-activity relationship and mechanism of action. Pharmaceuticals. 2023; 16:1–25. Doi: 10.3390/ph16040609
Oyeyemi IT, Akinseye KM, Adebayo SS, Oyetunji MT, Oyeyemi OT. Ethnobotanical survey of the plants used for the management of malaria in Ondo State, Nigeria. South African J. Bot. 2019; 124:391–401. Doi: 10.1016/j.sajb.2019.06.003
Rasoanaivo P, Wright CW, Willcox ML. Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J.2011; 10:1–12. Doi: 10.1186/1475-2875-10-S1-S4
Atanu FO, Idih FM, Nwonuma CO, Hetta HF, Alamery S, El-Saber Batiha G. Evaluation of antimalarial potential of extracts from Alstonia boonei and Carica papaya in Plasmodium berghei-infected mice. Evidence-based Complement. Altern. Med. 2021; 2021:1–11. Doi: 10.1155/2021/2599191
Ebhohimen IE, Edemhanrhia L, Ekozin A, Okolie NP. Effect of heat treatment on the antioxidant capacity of aqueous and ethanol extracts of Aframomum angustifolium seed. Trop. J. Nat. Prod. Res. 2017; 1:125–128.
Guide for the Care and Use of Laboratory Animals. (The National Academic Press, Washington, 2011). doi:https://doi.org/10.17226/12910.
Mbassi, E. F. A., Mombo-Ngoma, G., Ndoumba, W. N., Yovo, E. K., Eberhardt, K. A., Mbassi, D. E., Adegnika, A. A., Agnandji, S. T., Bouyou-Akotet, M. K., Ramharter, M., & Zoleko-Manego, R. Performance of Field’s Stain Compared with Conventional Giemsa Stain for the Rapid Detection of Blood Microfilariae in Gabon. Am. J. Trop. Med. Hyg. 2022; 107: 383–387. Doi: 10.4269/ajtmh.22-0061
Moody A. Rapid diagnostic tests for malaria. Clin Microbiol Rev. 2022; 15:66–78.
Feldman TP, Egan ES. Uncovering a Cryptic Site of Malaria Pathogenesis: Models to Study Interactions Between Plasmodium and the Bone Marrow. Front. Cell. Infect. Microbiol. 2022; 12:1–10. Doi: 10.3389/fcimb.2022.917267
Dasanna AK, Hillringhaus S, Gompper G, Fedosov DA. Effect of Malaria parasite shape on its alignment at erythrocyte membrane. Elife. 2021; 10:1–16. Doi: 10.7554/eLife.68818
Tarkang PA, Okalebo FA, Ayong LS, Agbor GA, Guantai AN. Anti-malarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models. Malar. J. 2014; 13:1–11. Doi: 10.1186/1475-2875-13-456
Obidike IC, Amodu B, Emeje MO. Antimalarial properties of SAABMAL®: An ethnomedicinal polyherbal formulation for the treatment of uncomplicated malaria infection in the tropics. Indian J. Med. Res 2015; 142:221–227. Doi: 10.4103/0971-5916.155585
White NJ. Anaemia and malaria. Malar. J 2018; 17:1–17. Doi: 10.1186/s12936-018-2509-9
Nwankwo N, Egbuonu ACC, Nduka FO, Nwodo OFC. Effect of seed extract of Picralima nitida on haematological parameters of malaria-infected albino mice and its interference with the serum electrolyte levels. Ife J. Sci. 2017; 19:379-387. Doi: 10.4314/ijs.v19i2.18
McKenzie FE, Prudhomme WA, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Wongsrichanalai C. White blood cell counts and malaria. J. Infect. Dis. 2005; 192:323–330. Doi: 10.1086/431152
Variability in white blood cell count during uncomplicated malaria and implications for parasite density estimation: a WorldWide Antimalarial Resistance Network individual patient data meta-analysis. Malar. J. 2023; 22:174. Doi: 10.1186/s12936-023-04583-6
Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C, Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar. J. 2014; 13:1–7. Doi: 10.1186/1475-2875-13-218