Effect of Moringa oleifera Leaf Extract on TGF-β1 and Galectin-3 Levels and Cardiac Fibrosis in Diabetic Rat
Main Article Content
Abstract
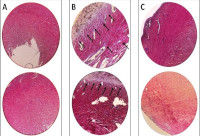
Cardiac fibrosis is the most prevalent form of diabetic cardiomyopathy. Increased levels of transforming growth factor-beta 1 (TGF-β1) and galectin-3 are present in cardiac fibrosis, and these proteins might be potential therapeutic targets. Researches have shown that Moringa oleifera (MO) display numerous biological activities which could be harnessed for the treatment of a variety of ailments, including cardiovascular diseases. This study is aimed at evaluating the effect of MO on TGF-β1 and galectin-3 levels in diabetic rat model of cardiac fibrosis. Fifteen (15) Wistar rats were randomly divided into three groups: normal control group, administered normal saline; case group, administered normal saline and streptozotocin; and MO treatment group, administered streptozotocin and MO extract (1000 mg/kg) once daily for 28 days. Cardiac fibrosis was evaluated by histopathological analysis of the rats’ myocardium. The levels of TGF-β1 and galectin-3 were investigated by enzyme-linked immunosorbent assay (ELISA). Histopathological examination revealed fewer cardiac fibrosis features in the myocardium of MO treated group compared to the case group. In addition, MO treatment resulted in a significant reduction in collagen decomposition in the left ventricle myocardium. ELISA revealed a significant decrease in the TGF-β1 and galectin-3 levels in the MO treated group compared to the case group (641.4±94.0 ng/L vs. 852.3±56.2 ng/L, and 1.53±0.07 ng/L vs. 1.79±0.166 ng/L, respectively). In conclusion, the beneficial effects of MO are likely related to its ability to decrease oxidative stress in the heart tissue and reduce the formation of fibrosis by suppressing the expression of TGF-β1 and galectin-3.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
1. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018; 379(7):633–644.
2. Benjamin, EJ., Virani, SS., Callaway, CW., Chamberlain, AM., Chang, AR., Cheng, S., Chiuve, SE., Cushman, M., Delling, FN., Deo, R., de Ferranti, SD., Ferguson, JF., Fornage, M., Gillespie, C., Isasi, CR., Jiménez, MC., Jordan, LC., Judd, SE., Lackland, D., Muntner, P. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018; 137(12):e67-e492.
3. From AM, Scott CG, Chen HH. The Development of Heart Failure in Patients With Diabetes Mellitus and Pre-Clinical Diastolic Dysfunction. J Am Coll Cardiol. 2010; 55(4):300–305.
4. Gutierrez PS and de Campos FPF. Endomyocardial fibrosis. Autopsy Case Rep. 2017; 7(3):3–6.
5. Li Y, Li T, Zhou Z, Xiao Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: A snapshot. Rev Endocr Metab Disord. 2022; 23(3):569–577.
6. Russo I and Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016; 90:84–93.
7. Walton KL, Johnson KE, Harrison CA. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front Pharmacol. 2017; 461(8):1-11.
8. Li YJ, Ji QQ, Wang Z, Shen LH, He B. Moringa oleifera seeds mitigate myocardial injury and prevent ventricular failure induced by myocardial infarction. Am J Transl Res. 2020; 12(8):4511–4521.
9. Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int J Mol Sci. 2015; 16(6):12791–12835.
10. Arbab AAI, Musa TH, Musa HH, Idris AA, Omer EAM, Oderinde OK, Akintunde YT. Mapping of Global 100 Top-cited Articles on Moringa oleifera Lam. from Documents Indexed in Web of Sciences Database. Trop J Nat Prod Res. 2022; 6(11):1778–1789.
11.Vergara-Jimenez M, Almatrafi M, Fernandez M. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants. 2017; 91(6):1-13.
12. Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020; 587(7835):555–566.
13. Mastikhina O, Moon BU, Williams K, Hatkar R, Gustafson D, Mourad O, Sun X, Koo M, Lam AYL, Sun Y, Fish JE, Young EWK, Nunes SS. Human cardiac fibrosis-on-a-chip model recapitulates disease hallmarks and can serve as a platform for drug testing. Biomaterials. 2020; 233:119741.
14. Watson CJ, Phelan D, Collier P, Horgan S, Glezeva N, Cooke G, Xu M, Ledwidge M, McDonald K, Baugh JA. Extracellular matrix sub-types and mechanical stretch impact human cardiac fibroblast responses to transforming growth factor beta. Connect Tissue Res. 2014; 55(3):248–256.
15. Villarruel-López A, Mora DAL de la, Vázquez-Paulino OD, Puebla-Mora AG, Torres-Vitela MR, Guerrero-Quiroz LA, Nuño K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement Altern Med. 2018; 127(18):1-10.
16. Li CJ, Lv L, Li H, Yu DM. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol. 2012; 73(11):1-10.
17. Ranjan P, Kumari R, Verma SK. Cardiac Fibroblasts and Cardiac Fibrosis: Precise Role of Exosomes. Front Cell Dev Biol. 2019; 318(7):1-12.
18. Raish M, Ahmad A, Jardan YAB, Shahid M, Alkharfy KM, Ahad A, Ansari MA, Abdelrahman IA, Al-Jenoobi FI. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed Pharmacother. 2022; 145:112412.
19. An Z, Yang G, Zheng H, Nie W, Liu G. Biomarkers in patients with myocardial fibrosis. Open Life Sci. 2017; 12(1):337–344.
20. Suryono S, Rohman MS, Widjajanto E, Prayitnaningsih S, Wihastuti TA. Colchicine as potential inhibitor targeting MMP-9, NOX2 and TGF-β1 in myocardial infarction: a combination of docking and molecular dynamic simulation study. J Biomol Struct Dyn. 2023; 41(21):12214-12224.
21. Suryono S, Rohman MS, Widjajanto E, Prayitnaningsih S, Wihastuti TA, Oktaviono YH. Effect of Colchicine in reducing MMP-9, NOX2, and TGF- β1 after myocardial infarction. BMC Cardiovasc Disord. 2023;449(23):1-10.
22. Dong J and Ma Q. In Vivo Activation and Pro-Fibrotic Function of NF-κB in Fibroblastic Cells During Pulmonary Inflammation and Fibrosis Induced by Carbon Nanotubes. Front Pharmacol. 2019; 1140(1):1-10.
23. Slack RJ, Mills R, Mackinnon AC. The therapeutic potential of galectin-3 inhibition in fibrotic disease. The Int J Biochem Cell Biol. 2021; 130:105881.
24. Madrigal‐Matute J, Lindholt JS, Fernandez‐Garcia CE, Benito‐Martin A, Burillo E, Zalba G, Beloqui O, Llamas-Granda P, Ortiz A, Egido J, Blanco-Colio LM, Martin-Ventura JL. Galectin‐3, a Biomarker Linking Oxidative Stress and Inflammation With the Clinical Outcomes of Patients With Atherothrombosis. J Am Heart Assoc. 2014; 3(4):e000785.
25. Inoue R, Kurahara LH, Hiraishi K. TRP channels in cardiac and intestinal fibrosis. Sem Cell Dev Biol. 2019; 94:40–49.
26. Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012; 23(4):705–715.
27. Lin BL, Matera D, Doerner JF, Zheng N, Del Camino D, Mishra S, Bian H, Zeveleva S, Zhen X, Blair NT, Chong JA, Hessler DP, Bedja D, Zhu G, Muller GK, Ranek MJ, Pantages L, McFarland M, Netherton MR, Berry A, Wong D, Rast G, Qian HS, Weldon SM, Kuo JJ, Sauer A, Sarko C, Moran MM, Kass DA, Pullen SS. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc Natl Acad Sci USA. 2019; 116(20):10156–10161.
28. She G, Hou MC, Zhang Y, Zhang Y, Wang Y, Wang HF, Lai BC, Zhao WB, Du XJ, Deng XL. Gal-3 (Galectin-3) and KCa3.1 Mediate Heterogeneous Cell Coupling and Myocardial Fibrogenesis Driven by βAR (β-Adrenoceptor) Activation. Hypertension (Dallas, Tex : 1979). 2020; 75(2):393–404.
29. Lin M, Zhang J, Chen X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J Func Foods. 2018; 47:469–479.
30. Panda S. Butanolic fraction of Moringa oleifera Lam. (Moringaceae) attenuates isoprotrenol-induced cardiac necrosis and oxidative stress in rats: an EPR study. EXCLI J. 2015; 14:64–74.
31. Gianosa G, Suryono S, Santosa A. Renoprotective Activity of Morinaga oleifera Lamk Toward Kidney Injury Rats Induced by Streptozotocin. NurseLine J. 2022; 7(1):21-27.
32. Salsabila ZM, Suryono, Pralampita PW. Protective effect of Moringa oleifera leaves extract on cardiac fibrosis of streptozotocin-induced diabetic rats. J Med Sci (Berkala Ilmu Kedokteran). 2023; 55(3):212-221.
33. Thongrung R, Pannangpetch P, Senggunprai L, Sangkhamanon S, Boonloh K, Tangsucharit P. Moringa oleifera leaf extract ameliorates early stages of diabetic nephropathy in streptozotocin-induced diabetic rats. J Appl Pharm Sci. 2023; 13(08):158–166.
34. Aly O, Abouelfadl DM, Shaker OG, Hegazy GA, Fayez AM, Zaki HH. Hepatoprotective effect of Moringa oleifera extract on TNF-α and TGF-β expression in acetaminophen-induced liver fibrosis in rats. Egypt J Med Hum Genet. 2020; 21(1):69.
35. Quagliariello V, Basilicata MG, Pepe G, De Anseris R, Di Mauro A, Scognamiglio G, Palma G, Vestuto V, Buccolo S, Luciano A, Barbieri M, Bruzzese F, Maurea C, Pumpo R, Ostacolo C, Campiglia P, Berretta M, Maurea N. Combination of Spirulina platensis, Ganoderma lucidum and Moringa oleifera Improves Cardiac Functions and Reduces Pro-Inflammatory Biomarkers in Preclinical Models of Short-Term Doxorubicin-Mediated Cardiotoxicity: New Frontiers in Cardioncology? J Cardiovasc Dev Dis. 2022; 423(9):1-22.