Synthesis, Biological Activity, and Computational Examination of New 3-Cyano-2-oxa-pyridine Derivatives http://www.doi.org/10.26538/tjnpr/v7i11.36
Main Article Content
Abstract
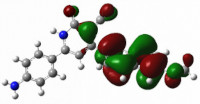
Numerous studies have been carried out into the chemistry of condensed heterocyclic compounds in terms of their medication discovery and various biological properties. Pyridines play an essential role in medicinal chemistry because they are widely available as natural compounds and have served as the foundation for several drugs on the market. In the current investigation, 3-cyano-2-oxa-pyridine derivatives 4a-e were synthesized by a one-pot multicomponent reaction, starting from substituted acetophenone, ethyl cyanoacetate, and aryl aldehydes in the presence of ammonium acetate. All the new products were subjected to proton nuclear magnetic resonance (1H NMR), carbon nuclear magnetic resonance (13C NMR), two-dimensional (2D)-NMR analysis using heteronuclear single quantum coherence spectroscopy (HSQC), and electron ionization (EI-MS). Additionally, an in vitro cytotoxicity test was performed on cervical carcinoma (HeLa) and cerebral glioblastoma multiforme (AMGM5) cells for every produced molecule. The results indicated that the tested compounds 4a, 4c, and 4e inhibited AMGM5 cells with average IC50 values of 656.4, 781.5, and 374.5 μM, respectively. Compounds 4a, 4b, and 4e, on the other hand, showed a cytotoxic action against the HeLa cell line, with average IC50 values of 558.5, 775.6, and 615.9 μM, respectively. The optimized geometry and reactivity descriptors were also analyzed, including the highest occupied molecular orbital (HOMO), least unoccupied molecular orbital (LUMO), energy band gap (ΔE), chemical potential (µ), electronegativity (χ), chemical hardness (η), chemical softness (S), and electrophilicity (ω). The experimental outcomes of the biological evaluation were consistent with the results of the investigation into their molecular modeling.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Hovhannisyan AA, Aristakesyan LH, Hakobyan RM, Melikyan GS. Synthesis of new 3-cyanopyride-2(1H)-ones with unsaturated substituents AT C-4. Proc. of the Yerevan State Univ. Chem. Biol. 2015; 2: 9–13.
Pareshkumar UP, Vipul PG, Purohitb DM, Patolia VN. Synthesis and biological evaluation of some new cyano pyridine derivatives. J. Chem. Pharm. Res. 2015; 7(1):182-186.
Ahmed M, Mona M, Hassan A, Ola R, Omima S, Mohamed S, Zaki AS, Hayam A. New 3-Cyano-2-Substituted Pyridines Induce Apoptosis in MCF 7 Breast Cancer Cells. Mol. 2016; 21: 230.
Azzarito V, Long K, Murphy NS, Wilson AJ. Inhibition of α-helix-mediated protein–protein interactions using designed molecules. Nat Chem. 2013; 5(3): 161-173.
Marzouk AA, Bass AK, Ahmed MS, Abdelhamid AA, Elshaier YA, Salman AM. Design, synthesis and anticonvulsant activity of new imidazolidindione and imidazole derivatives. Bioorg. Chem. 2020; 101: 104020.
Murata T, Shimizu K, Narita M, Manganiello VC, Tagawa T. Characterization of phosphodiesterase 3 in human malignant melanoma cell line. Anticancer Res. 2002; 22(6A): 3171-3174.
Teague SJ. Synthesis of heavily substituted 2-aminopyridines by displacement of a 6-methylsulfinyl group. J. Org. Chem. 2008; 73(24): 9765-9766.
Bass AKA, El-Zoghbi MS, Abdelhafez EMN, Mohamed MF, Badr M, Abuo-Rahma GE-DA. Comprehensive review for anticancer hybridized multitargeting HDAC inhibitors. Eur. J. Med. Chem. 2021; 209: 112904.
Bringmann G, Reichert Y, Kane VV. The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 2004; 16(60): 3539-3574.
Zhou Y, Kijima T, Kuwahara S, Watanabe M, Izumi T. Synthesis of ethyl 5-cyano-6-hydroxy-2-methyl-4-(1-naphthyl)-nicotinate. Tetrahedron Lett. 2008; 49(23): 3757-3761.
Faidallah HM, Rostom SA, Badr MH, Ismail AE, Almohammadi AM. Synthesis of Some 1, 4, 6‐Trisubstituted‐2‐oxo‐1, 2‐dihydropyridine‐3‐carbonitriles and Their Biological Evaluation as Cytotoxic and Antimicrobial Agents. Arch. Pharm. Chem. Life Sci. 2015; 348: 824-834.
Al-Etaibi AM, El-Apasery MA. A comprehensive review on the synthesis and versatile applications of biologically active pyridone-based disperse dyes. Int. J. Environ. Res. Public Health. 2020; 17(13): 4714.
Mamedov I, Naghiyev F, Maharramov A, Uwangue O, Farewell A, Sunnerhagen P, et al. Antibacterial activity of 2-amino-3-cyanopyridine derivatives. Mendeleev Commun. 2020; 30: 498-499.
Hanaa MH, Mohamed MA. Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharm. 2008; 58(2): 175–186.
Al‐Omar MA, Amr AEGE, Al‐Salahi RA. Anti-inflammatory, analgesic, anticonvulsant and antiparkinsonian activities of some pyridine derivatives using 2,6-disubstituted isonicotinic acid hydrazides. Arch. Pharm. Chem. Life Sci. 2010; 343: 648-656.
Amr AEGE, Sayed HH, Abdulla MM. Synthesis and reactions of some new substituted pyridine and pyrimidine derivatives as analgesic, anticonvulsant and antiparkinsonian agents. Arch. Pharm. Chem. Life Sci. 2005; 338: 433-440.
Ismail MM, Farrag AM, Harras MF, Ibrahim MH, Mehany AB. Apoptosis: A target for anticancer therapy with novel cyanopyridines. Bioorg. Chem. 2020; 94: 103481.
Eman M. Flefel, Hebat-Allah S. Abbas, Randa E. Abdel Mageid and Wafaa A. Zachary, Synthesis and Cytotoxic Effect of Some Novel 1,2-Dihydropyridin-3-carbonitrile and Nicotinonitrile Derivatives. Mol. 2016; 21(1); 30.
Ahmed HS, Eman RK, Manal MA, Mohamed S. A Review on The Chemistry of Nicotinonitriles and Their applications. Egypt. J. Chem. 2021; 64: 4509 – 4529.
Ali AAA, Khalid ASA, Ahmed MA. Cytotoxic effects of CeO2 NPs and β- carotine and their ability to induce apoptosis in human breast normal and cancer cell lines. Iraqi J. Sci. 2022; 63: 3.923-937.
Falih SM, Al-Saray ST, Alfaris AA, Al-Ali A.A. The synergistic effect of eucalyptus oil and retinoic acid on human esophagus cancer cell line SK-GT-4. Egypt. J. Med. Hum. Genet. 2022; 23:70.
Al-Shammari AM, Al-Esmaeel WN, Al-Ali AA, Hassan AA, Ahmed AA. Enhancement of Oncolytic Activity of Newcastle Disease Virus Through Combination with Retinoic Acid Against Digestive System Malignancies. Mol. Ther. 2019; 27(4S1):126-127
Freshney RI. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. 6th ed. Wiley-Blackwell. 2010; P732.
Saima M, Rahat A, Sumreen H, Muhammad UI, Asma A, Nimrah Z, Shabana N, Mubashera S. Integrating In Silico and In Vitro Approaches to Screen the Antidiabetic Properties from Tabernaemontana divaricata (Jasmine) Flowers. Evid. Based Complement. Alternat. Med. 2022; 9: 1-17.
Anees P, Khursheed A. Synthesis and biological evaluation of coumarin-quinone hybrids as multifunctional bioactive agents. Admet Dmpk. 2023; 11(1): 81-96.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ. Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016.
Akbar M, Sajad A, Mohammad AB. Convenient, multicomponent, one-pot synthesis of highly substituted pyridines under solvent-free conditions. Synth. Commun.. 2016; 1-16.
Sobhi MG, Zeinab AM, Mohamad RA, Hassan MA, Hatem MG, Mahmoud ME. One-Pot Synthesis of New Thiadiazolyl-Pyridines as Anticancer and Antioxidant Agents. J. Heterocyclic Chem. 2018; 55(2): 530-536.
Amr KAB, Elshimaa MNA, Mona SE, Mamdouh FAM, Mohamed B, Gamal El-Din AAR. 3-Cyano-2-oxa-pyridines: a promising template for diverse pharmacological activities. J. Adv. Biomed. Pharm. Sci. 2021; 4: 81-86.
Mohammed A, Hassan AA. Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arab. J. Chem. 2022; 15(6): 103846.
Sourav De, Ashok KSK, Suraj KS, Sabnaz K, Nandan S, Subhasis B, Sanjay D. Pyridine: the scaffolds with significant clinical diversity. RSC. Adv. 2022; 12: 15385-15406.
Alaa MA, Abrar AB. Synthesis and antiproliferative activity studies of new functionalized pyridine linked thiazole derivatives. Arab. J. Chem. 2021; 14: 102914.
Sahu R, Mishra R, Kumar R, Chandana MS, Mazumder A, Kumar A. Pyridine Moiety: Recent Advances in Cancer Treatment. Indian J. Pharm. Sci. 2021; 83(2): 162-185.
Yong L, Zhi-YH, Dong L, Chun-LZ, Yan-FL, Yan W. The Expanding Role of Pyridine and Dihydropyridine Scaffolds in Drug Design. Drug Des. Devel. Ther. 2021; 15: 4289.
Lynda G, Rachid C, Mohammed L, Paul M. Synthesis, characterization of some substituted Quinolines derivatives: DFT, computational, in silico ADME, molecular docking and biological activities. Chem. Data Collect. 2023; 43:100977.
Ayesha N, Faisal A O, Asia N A, Aqeel I, Abdul H, Syed A A S, Jamshed I, Zainul A Z. Exploring Novel Pyridine Carboxamide Derivatives as Urease Inhibitors: Synthesis, Molecular Docking, Kinetic Studies and ADME Profile. Pharm. 2022; 15: 1288.
Mohamed R, Asmaa O, Halima H, Burak T, Abdelhadi H, El Hassane A, Elyor B, Mohammed A A, Abdelkader Z, Brahim L. Synthesis, bioinformatics and biological evaluation of novel pyridine based on 8-hydroxyquinoline derivatives as antibacterial agents: DFT, molecular docking and ADME/T studies. J. Mol. Struct. 2021; 1244: 130934.
Yong L, Zhi-YH, Dong L, Chun-LZ, Yan-FL, Yan W. The Expanding Role of Pyridine and Dihydropyridine Scaffolds in Drug Design. Drug Des. Devel. Ther. 2021; 15: 4289.
Gökhan G, Semra B. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 2009; 51(8): 1876–1878.
Jun-ichi A. Correlation found between the HOMO–LUMO energy separation and the chemical reactivity at the most reactive site for isolated-pentagon isomers of fullerenes. Phys. Chem. Chem. Phys. 2000; 2(14):3121-3125.
Marzieh M, Abolfazl S, Khalil P, Ahmad RO, Farhad H. Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo[d] thiazole-2(3H)-imine and its para-substituted derivatives: Solvent and substituent effects. J. Chem. Res. 2021; 147–158.
David P, Jyotirmoy D, Christian VA, Utpal S. Theoretical Investigation of Electronic, Vibrational and Nonlinear Optical Properties of 4-fluoro-4- hydroxybenzophenone. Spectrosc. Lett. 2017; 50: 4.
Selma ŠH, Mirsada S, Hurija DČ, Snezana T, Suncica R, Dzenita S, Davorka Z. DFT study and microbiology of some coumarin-based compounds containing a chalcone moiety. J. Serb. Chem. Soc. 2014;79 (4): 435–443.
Cihat H, Müşerref Ö, Mehmet EM. Detonation Parameters of the Pentaerythritol Tetranitrate and Some Structures Descriptors in Different Solvents - Computational Study. Düzce University J. Sci. Technol. 2021; 9: 1227-1241.
Richard M, Lo P, Terrence G. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014; 27(7): 1081–1091.
Chattaraj PK, Arun Murthy TVS, Giri S, Roy DR. A connection between softness and magnetizability. J. Mol. Struct. 2007; 813: 63-65.
Andrew RJ, Timothy CJ, Douglas WS. The global electrophilicity index as a metric for Lewis acidity. Dalton Trans. 2018; 47: 7029-7035.
Al Shuhaib Z, Hussein K A, Ismael S M. Synthesis of New Pyrimidine Derivatives, Study of Anti-Сancer Activity, Structural Properties, and Molecular Docking. Russian J. Gen. Chem. 2023; 93(5): 1171–1180.
Mar R-G, Alejandro S S, Luis R D. Electrophilicity and nucleophilicity scales at different DFT computational levels. J. Phys. Org. Chem. 2023; 36: e4503.
Luis RD, Mar RG, Patricia P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Mol. 2016; 21(6): 748.